Fractional Yellow Fever Dosing Works, But Not For International Travelers

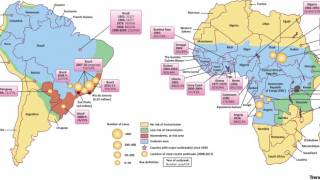
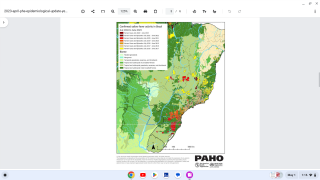
The yellow fever virus has now surfaced at the doorstep of urban areas on the Atlantic ocean rim.
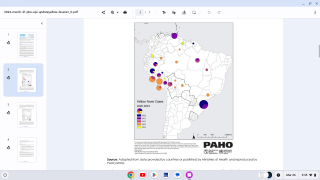
Currently, there is a large, ongoing outbreak of yellow fever in Brazil, including the cities of Rio de Janeiro and São Paulo.
A number of unvaccinated international travelers have gotten yellow fever, and several have died (none from the United States).
In an editorial letter excerpted below, three researchers discuss fractional dosing and yellow fever vaccination requirements for international travel.
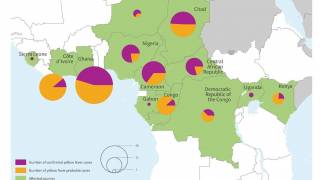
In 2016, a global shortage of yellow fever vaccine occurred as a result of major yellow fever outbreaks in Angola and the Democratic Republic of Congo (DRC). By October, 7136 cases and 493 deaths were reported in the two countries.
In Kinshasa, the capital of the DRC, a preventive campaign targeting roughly 10.5 million people was needed to mitigate the risk of an urban yellow fever outbreak.
However, only 5.8 million vaccine doses were available from the World Health Organization (WHO) stockpile.
A solution was urgently needed.
Faced with the options of using off-label fractional-dose vaccine to meet the supply needs or using the full-dose vaccine but leaving millions of people at risk for yellow fever, the DRC, in close consultation with the WHO, opted to use one fifth of the standard 0.5 ml volume of vaccine (0.1 ml) in its vaccination campaign.
More than 7 million people received the fractional-dose vaccine in Kinshasa in August 2016.
This decision was based on simple math.
WHO-prequalified yellow fever vaccines are highly potent, with average doses between 12,874 and 43,651 international units (IU) — far above the WHO’s recommended minimum of 1000 IU.
In principle, the quantity of vaccine virus in fractional doses of the standard vaccine would therefore still exceed the WHO’s minimum requirement.
But fractionating yellow fever vaccine doses is not without complexity.
Average doses vary substantially among vaccine manufacturers and among product batches from a given manufacturer. Fractionating doses from vaccine vials at the lower end of the dosage range could result in doses close to or below the WHO minimum.
Furthermore, potency can wane when vaccines are nearing the end of their shelf life, which for WHO-prequalified yellow fever vaccines is 36 months.
Even the WHO’s recommended minimum dose must be regarded with some caution since it is based on studies in animals rather than rigorous dose-finding studies in humans.
Thus, it is important to ensure that the immunogenicity of fractional doses is equivalent to that of standard doses of currently used vaccines.
At the time of the 2016 outbreak, there were three publications from two studies on the safety and immunogenicity of fractional-dose yellow fever vaccine administered through the recommended route (intramuscular or subcutaneous).
Although these data were considered reassuring, they were restricted to a single country, manufacturer, and population (male adults).
Additionally, a randomized non-inferiority trial was recently launched comparing seroconversion after fractional-dose and full-dose yellow fever vaccination for each WHO-prequalified vaccine product. This study will evaluate fractional-dose vaccination in adults living with HIV and in children.
In the context of the emergency situation and vaccine shortage, the WHO considered these data sufficient to proceed with a fractional-dose vaccination campaign.
Full-dose vaccination was still recommended for young children and pregnant women.
The WHO published its official position on fractional-dose yellow fever vaccination in June 2017. The agency recommends fractional-dose vaccination during a yellow fever outbreak only if there is a shortage of full-dose vaccine and emergency-response needs exceed the capacity of the global stockpile.
Furthermore, the WHO still recommended that some groups, such as children less than 2 years of age and pregnant women, receive the full-dose vaccine, given the lack of data demonstrating the safety and immunogenicity of fractional doses.
Because of limited data on duration of protection, fractional-dose vaccination also does not qualify people for international travel under the International Health Regulations.
Without assurances that a fractional-dose vaccine provides the same lifetime protection as a full-dose vaccine, people who receive fractional doses will need to be revaccinated before traveling to countries where yellow fever is endemic and where the International Health Regulations require proof of vaccination.
The US Centers for Disease Control and Prevention (CDC) says the yellow fever vaccine is the best protection against yellow fever disease, which can be fatal.
Because of a total depletion of their supply of YF-Vax, the manufacturer (Sanofi Pasteur) has worked with CDC and the US Food and Drug Administration to make an alternative yellow fever vaccine, Stamaril, available at select locations until YF-Vax supply returns, which is expected by the end of 2018.
To research, if yellow fever vaccine is needed at your international travel destination, pls visit this CDC page.
Only a limited number of clinics in the United States have the vaccine. To schedule a travel vaccination appointment, please visit Vax-Before-Travel.
The CDC Vaccine Price List provides private sector vaccine prices for general information. And, vaccine discounts can be found here.
Vaccines, like any medicine, can have side effects, says the CDC. You are encouraged to report negative side effects of vaccines to the FDA or CDC.
The views expressed in this editorial article are those of the authors and do not necessarily represent the decisions or policies of the World Health Organization. Dr. Vannice was on staff at the WHO during the development of its policy on fractional-dose vaccination.
Here are the disclosure forms provided by the authors.
This article was published on July 11, 2018.
Our Trust Standards: Medical Advisory Committee