Beware of fake Duo-cotecxin medication on Tanzanian market: TFDA
What you need to know:
- TFDA says the fake tabs were manufactured by a Chinese company under delivery number 170259 with manufacturing date: February25 2017 and expiry date February 2019.
- The fakes, were found in 22 packs and 9 tablets at two drug shops in Kinondoni Mkwajuni suburb, Dar es Salaam during an inspection.
Dar es Salaam. If you fall sick and your doctor recommends tablets by the name Duo-cotecxin—a commonly used antimalarial drug—take extra precautions because there is a fake version of the same medication on the market.
Tanzania Food and Drugs Authority (TFDA) has warned of the presence of a fake batch of the tablets in drug shops and pharmacies in the country and issued guidelines on what steps one can take.
TFDA says the fake tabs were manufactured by a Chinese company under delivery number 170259 with manufacturing date: February25 2017 and expiry date February 2019.
The fakes, were found in 22 packs and 9 tablets at two drug shops in Kinondoni Mkwajuni suburb, Dar es Salaam during an inspection. The owners of the drug shops are being interrogated by police, TFDA said in a statement.
About 100,000 deaths a year in Africa are linked to the counterfeit drug trade, according to the World Health Organization (WHO).
The British think-tank, International Policy Network, estimates that globally, 700,000 deaths a year are caused by fake malaria and tuberculosis drugs—comparing the death toll to the equivalent of “four fully laden jumbo jets crashing everyday.”
Following this alarm, the TFDA has advised that patients whose prescription includes Duo-cotecxin to inspect the pack for any indications that it could be fake.
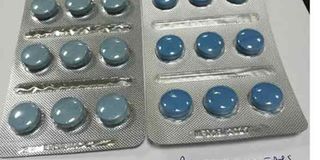
TFDA says the fakes are easy to recognize because they do not show the expiry date in the primary pack inside, contrary to the genuine ones which show.
But also, the registration details of the fake version of the medications are not indicated in proper order on the secondary pack (outside) and its package insert does not have its English version, TFDA has further warned.
“By comparison, the fake tablets are light blue in colour while the genuine ones are dark blue,’’ TFDA said.
“If one examines the pack, one will realize that the inside pack of the fake tablets bears delivery number 150644 which is different from the outer pack with number 170259. But for the genuine tablets, the delivery number is the same both inside and outside,’’ said TFDA.
All health workers and drug shop owners have been asked to report to TFDA in case they spot the fake medications on the market.
Duo-cotexin in news yet again
This is not the first time that Duo-cotecxin medication has been in the news for the wrong reasons. In December last year, The Citizen’s sister newspaper in Kenya, Daily Nation reported that a certain version of Duo-Cotecxin had been recalled from the Kenyan market for failing a laboratory quality test. Kenya’s Health ministry took action.
That followed an investigation by Daily Nation in collaboration with the Code for Africa impact AFRICA Fund, which revealed that the drug of batch number 160621, which was widely in circulation across the country, failed an assay test, making it substandard and fake.
The drug samples that were purchased from a pharmacy outlet in Nairobi were said to be manufactured by the sane Chinese company and were due for expiry in May 2018.
Africa ruined by fake medications
Last year, the World Health Organization (WHO) released a report showing that one in every 10 medical products in low and middle-income countries – including Tanzania – is substandard or falsified, meaning that people are taking medicines that are not working.
The WHO defines counterfeit/fake medicine as “one which is deliberately and fraudulently mislabeled with respect to identity and/or source.” Both branded and generic products are faked.
In some parts of Africa, Asia and Latin America, more than 30 percent of the medicines on sale can be fake, notes the organization