Malaria Vaccine Candidate Passes First Human Phase 1 Study

A team of researchers at the University of Copenhagen have been focussing on developing a vaccine that can protect against the disease ‘Pregnancy Associated Malaria’ (PAM), from which 220,000 people die every year.
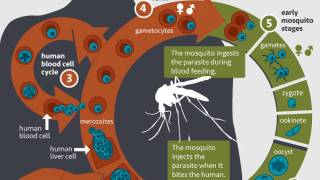
In addition to an effect on low birth weight and prematurity, PAM determines fetal exposure to Plasmodium falciparum in utero and is correlated to congenital malaria and early development of clinical episodes during infancy.
According to this team, they have come one step closer to being able to introduce such a preventive vaccine in the market.
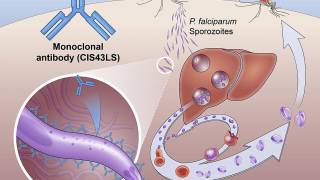
These researchers developed a malaria vaccine candidate aiming at reducing sequestration of asexual “blood-stage” parasites in the placenta, the major virulence mechanism in PAM.
This is important news since 2018 report showed that after an unprecedented period of success in controlling malaria outbreaks, progress has stalled.
The World Malaria Report 2018 aggregated data from 91 countries and areas with ongoing malaria transmission.
This World Health Organization (WHO) report says there were an estimated 219 million malaria cases and 435,000 related deaths in 2017.
This new study published in the scientific journal Clinical Infectious Diseases reported the Phase 1 clinical trial of 36 women and men found the vaccine safe to use.
After injecting the test subjects with the vaccine, the researchers were able to detect the right immune response with antibodies against the malaria parasite in the blood, and the subjects showed no serious side effects.
'It is a great milestone for us to be able to show that our vaccine is completely safe and induces the exact antibody response in the blood we want. Because it is the immune response that has been shown to be connected with protection from pregnancy malaria.'
The next step is to document that it prevents pregnancy malaria in African women who would otherwise have contracted the disease before first pregnancies, said Associate Professor Morten Agertoug Nielsen, in a press release.
The effect of the vaccine was examined among 36 'malaria naïve' German women and men who had volunteered for the trial.
'The next step in the process is a phase 2 clinical trial, which will show whether the vaccine is still safe, but also whether it can prevent disease.’
‘Concurrently, we have developed a method for transforming the vaccine into a virus-like particle. This increases the antibody response.’
‘But, the crux of the matter is whether it is sufficient for attacking all the different forms of the protein hook found in the malaria parasite', said Associate Professor Morten Agertoug Nielsen.
The University of Copenhagen owns the patent on the vaccine technology. In this study, the researchers have also worked closely together with researchers at Tübingen University Hospital and the German Center for Infection Research.
These researchers disclosed various industry relationships.
The study is funded by the EU programme FP7-Health-2012-Innovation, the Danish National Advanced Technology Foundation, the Independent Research Fund Denmark, the German Federal Ministry of Education and Research the Bill & Melinda Gates Foundation.
Our Trust Standards: Medical Advisory Committee
- First-in-human, randomized, double-blind clinical trial of differentially adjuvanted PAMVAC, a vaccine candidate
- Pregnancy-associated malaria and malaria in infants: an old problem with present consequences
- Safety and Immunogenicity of the Placental Malaria Vaccine Candidate PAMVAC Variously Adjuvanted (PAMVAC)
- Malaria Makes A Worldwide Comeback