In December 2015 then president Benigno Aquino III of the Philippines and others negotiated a deal with pharmaceutical company Sanofi to purchase three million doses of Dengvaxia, the first vaccine ever licensed for dengue. The plan was to give a million schoolchildren, nine years of age, three doses of the vaccine each, sparing them from the worst outcomes of dengue: shock, organ failure and death.
The virus comes in four varieties. All are spread by female Aedes mosquitoes, primarily Aedes aegypti, with a penchant for sucking blood during the day, when individuals are unprotected by bed nets. In the past five decades these viruses, which are related to those that cause West Nile fever, yellow fever and Zika, have spread in waves across the tropical and subtropical world, increasing dengue incidence 30-fold and affecting upward of 390 million people each year.
Not everyone infected with a dengue virus gets sick: three out of four who get bitten will have no symptoms. The rest may suffer one of three sets of symptoms: a fever that mimics many other viral illnesses; “dengue fever,” which is accompanied by headache, pain behind the eyes, aching joints and bones, and, in rare cases, internal bleeding; and severe disease encompassing dengue hemorrhagic fever and dengue shock syndrome. In severe cases, plasma seeps out of capillaries, liquid pools around organs, massive internal bleeding ensues, and the brain, kidneys and liver begin to fail. Although swift hospitalization and careful case management can and do save lives, more than 20,000 people die of dengue every year. Many are children.
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing. By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.

Child in Managua, Nicaragua, yields a blood sample (left) for an extensive study of dengue disease. Another child (right) looks down his neighborhood street. Credit: Paolo Harris Paz
Dengue is scary enough that health practitioners in developing countries have been eagerly awaiting a vaccine for decades. Yet when internist Antonio Dans and pediatrician Leonila Dans, both clinical epidemiologists at the University of the Philippines Manila College of Medicine, read about Aquino’s vaccination campaign in the Philippine Star, the first thing that struck them was the price tag. At three billion pisos ($57.5 million) for procurement alone, the Dengvaxia campaign would cost more than the entire national vaccination program for 2015, which covered pneumonia, tuberculosis, polio, diphtheria, tetanus, pertussis, measles, mumps and rubella. It would reach less than 1 percent of the country’s approximately 105 million residents. And although dengue was reported to kill an average of 750 people annually in the Philippines, it was not even among the top 10 causes of mortality. Among infectious diseases, pneumonia and tuberculosis took a far heavier toll.
Perusing an interim report from researchers at Sanofi Pasteur—the vaccine division of Sanofi—on Dengvaxia’s clinical trials, Dans and Dans found further cause for concern. Among Asian children two to five years old, those who had received the vaccine were seven times more likely than unvaccinated children to have been hospitalized for serious dengue in the third year after vaccination. Close examination of the data revealed that although the vaccine was on average safer for older children, it was statistically impossible to rule out the possibility that for some kids, Dengvaxia made things worse.
In March 2016 Dans and Dans and other medical professionals wrote to then secretary of health Janette Garin, warning that the vaccine could be risky for some children and that the Philippines may not possess enough trained health care workers to monitor so many of them for possible adverse effects. A potentially safer vaccine was in the pipeline and probably worth waiting for, they reasoned.
The same month, however, the highly respected advisory group on vaccines at the World Health Organization—which provides guidance to countries on immunization policy—stated in a briefing paper on Dengvaxia that the hospitalizations of young vaccinated children, when observed over several years, were not statistically significant. “No other safety signals have been identified in any age group” older than five, it stated. A “theoretical possibility” existed that the vaccine could be risky for some children, and further research was necessary lest the issue “compromise public confidence” in the vaccine. It nonetheless “should be introduced as part of a routine immunization program in appropriate settings.” These included regions where 70 percent or more of a population had already had dengue, where immunization of early adolescents could reduce hospitalizations by up to 30 percent over a period of 30 years. A subsequent position paper from the same group stated that the vaccine was safe for children age nine and older, for whom it was recommended.
In retrospect, it did not surprise Dans and Dans that the authorities chose to ignore their concerns. “It was either believe us or believe the WHO,” says Antonio Dans. “If I were them, I’d believe the WHO. I mean, who were we? We were just teachers in a small medical school.” Filipino authorities were apparently so confident about Dengvaxia’s safety that they did not oblige Sanofi Pasteur to submit results from so-called pharmacovigilance trials that would usually test the safety of a new drug or vaccine in local conditions. The induction of a new pharmaceutical product into the national program typically took three to five years, says Anthony Leachon, a former president of the Philippine College of Physicians, but the dengue vaccination program began right away, in April 2016.
Days later came the first report of a postvaccination fatality, of a boy with congenital heart disease. Garin explained in a press briefing that the boy’s death was unrelated to Dengvaxia. Dans and Dans persisted for months, however, speaking to the press and posting a brief video on Facebook that warned—on the basis of a decades-old, highly contested theory called antibody-dependent enhancement (ADE)—that if a child had never had dengue before, the vaccine might actually make a dengue infection deadlier than it normally would have been. Garin responded with her own warning: medical practitioners who engaged in “misinformation” on Dengvaxia would be responsible for every death from dengue that could have been prevented by the vaccine.
There the matter rested until November 2017, when Sanofi Pasteur issued its own advisory: those who had never experienced a dengue infection should not get Dengvaxia. A month later the WHO issued fresh guidelines, recommending the vaccine only for those with a “documented past dengue infection.” The Philippines halted the vaccination program that December even as parents and the press responded with fury, recriminations and further reports of children’s deaths. More than 830,000 schoolchildren had been vaccinated. According to the Department of Health (DOH), as of September 2018, 154 of the vaccinated children had died of various illnesses. The vast majority of these fatalities were unrelated to the vaccine, but clinical observations or blood tests confirmed that 19 of them had been caused by dengue.
Sanofi Pasteur contends that the deaths in the Philippines could have arisen from a failure of the vaccine to protect a small fraction of those vaccinated. In contrast, some experts argue, as Dans and Dans did, that Dengvaxia mimics a prior encounter with dengue—which can prime a patient’s body to respond in a dangerous way to a second dengue infection.
The controversy has not slowed down the rollout of Dengvaxia, which is currently licensed in more than 20 countries. In October 2018 the U.S. Food and Drug Administration announced that it would prioritize review of Sanofi Pasteur’s application to approve Dengvaxia. That means it could be approved in the U.S., for use in dengue-endemic areas such as Puerto Rico, before the Philippines completes its investigation into the deaths of vaccinated children—and before Sanofi Pasteur publishes its final report from the six-year-long clinical trials.
Credit: Tami Tolpa
A baffling disease
For most viruses, such as measles, the second bout, if it occurs at all, is much milder than the first. For dengue, a second bout is far more likely to kill. Scientists and doctors have struggled for years to understand why this is so. In the 1950s and 1960s, when epidemics of severe dengue began to rise in Asia, they wondered if they were dealing with an altogether new infection. The dengue they were familiar with kept patients bedridden and fatigued, but this new manifestation sent them to the hospital or the morgue. Had the virus mutated? Or was the immune system to blame?
A young scientist fresh out of medical school was seeking an answer. Scott B. Halstead began to study mosquito-borne viruses in 1957, while working for the U.S. Army in Japan. He confronted his first major dengue outbreak four years later, when stationed at a military laboratory next door to the Bangkok Children’s Hospital. Doctors thought the youngsters who were carried into the hospital had been poisoned; almost a quarter of them died. Halstead led the team that identified dengue as the cause of the outbreak. He went on to make a second, more baffling, discovery. Children who were infected with dengue for a second time—each time with a different dengue virus—and babies born to mothers who were immune to dengue were most at risk for severe dengue and death. No one could explain why.
In 1964 R. A. Hawkes, then a researcher at Australian National University in Canberra, found that cell cultures infected with Murray Valley encephalitis, West Nile, Japanese encephalitis or Getah viruses infected more cells when the virus was mixed with antibodies compared with the virus alone. Hawkes proposed that the antibodies were stabilizing the virus and increasing their ability to attach to cells. Independently, Halstead was wondering if much the same was happening with dengue.
To understand why two different dengue infections were needed to make the second one lethal, Halstead infected 118 monkeys with different combinations of the four dengue viruses and measured the amount of virus in their blood. In 1973 he published his results: some monkeys, which were infected a second time and with a different dengue virus, had much higher viral loads. Four years later he provided a possible explanation, calling it antibody-dependent enhancement.
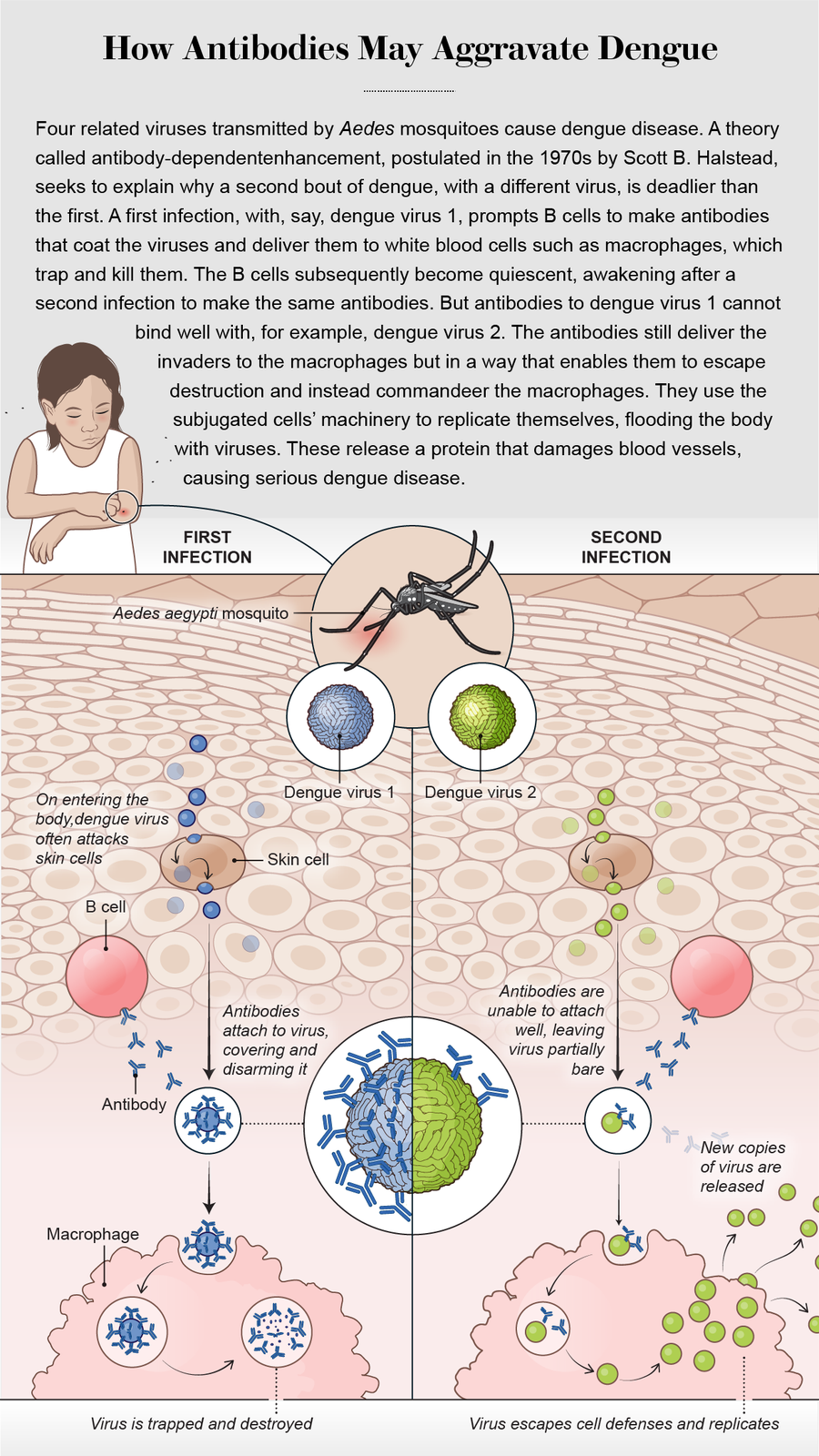
Say your first infection is with the dengue virus called DENV-1. Antibodies against that virus can linger in your blood for decades, even your entire life. When you are infected a second time with a different dengue virus, say DENV-2, 3 or 4, the antibodies against DENV-1 could paradoxically accelerate the replication of the new virus inside infected cells, precipitating a potentially fatal dengue infection.
Since refined by Halstead and other researchers, the ADE mechanism goes as follows: A dengue virus is a string of ribonucleic acid enclosed in a protein capsule, which features an array of characteristic protuberances on its surface. During a first infection with dengue, the immune system’s B cells make an antibody called immunoglobulin G, or IgG, which latches onto one or more of these irregularities. On attachment, the antibodies can deliver the virus to immune system cells such as macrophages. The word “phage” derives from the Greek word meaning “to eat”: macrophages are literally “big eaters.” They engulf the virus and digest it with enzymes. Thus, once it is bound to antibodies, the dengue virus is normally trapped and destroyed inside macrophages.
When an infection is over, some antibody-making B cells become dormant. In the event of a second infection with a different dengue virus, these cells wake up to churn out the exact same antibodies as before. Halstead postulated that some of these antibodies can still stick to the surface of the unfamiliar virus but often fail to block its most lethal protrusions—its guns, so to speak. The antibodies still deliver the intruder to macrophages but without having disarmed it. That enables the virus to immobilize the macrophage’s own defense system and take over the cell, whose resources it then uses to churn out more copies of itself. The antibody’s unwitting assistance helps the new dengue variety produce 1,000 times more copies of itself than if it were acting alone.
Halstead’s reward for coming up with the ADE hypothesis was a mix of indifference or disbelief from his peers, he recalls. Today, at 89 years old, he is an adjunct professor at the Uniformed Services University of the Health Sciences in Bethesda, Md., where he continues to argue his case. Many dengue experts describe him as the Godfather of ADE. “Back then, I was thinking I’ve made a discovery that’s very important,” he says. “Except nobody wanted to believe ADE was real.”
More than four decades later Eva Harris, a dengue expert at the University of California, Berkeley, found strong evidence that ADE was not only real but that it contributed to severe dengue disease in children. Harris had not set out to prove or disprove ADE: she was initially skeptical of the phenomenon and not all that keen on engaging in the decades-long debate. Instead her team, including statistical modeler Leah Katzelnick, was studying the ways in which dengue sickens children. That goal then led the researchers to help establish a lab in Nicaragua and to begin one of the more challenging types of scientific projects: a long-term pediatric cohort study. Harris and her associates in Managua, Nicaragua’s capital city, had the not so easy task of following thousands of children.
For more than 15 years the scientists working on the Nicaraguan Pediatric Dengue Cohort Study cared for the children if they got sick and went to their homes to collect data and blood samples. Out of 6,684 subjects, the researchers found 618 who had been sick with dengue and nearly four dozen who developed severe disease. Scouring more than 41,000 blood samples, taken over more than a dozen years, they made a striking discovery. Children with a specific concentration of antibodies—not low enough to be useless, not high enough to offer protection, but a concentration of antibodies in a middling range—were at a nearly eight times higher risk of acquiring dengue hemorrhagic fever and dengue shock syndrome.
.png?w=900)
Credit: Amanda Montañez; Source: “Effect of Dengue Serostatus on Dengue Vaccine Safety and Efficacy,” by Saranya Sridhar et al., in New England Journal of Medicine, Vol. 379, No. 4; July 26, 2018
ADE handily explains this finding. If the antibodies are not there to begin with or are present at very low densities, they cannot enhance a subsequent dengue infection to cause serious disease. If antibodies are present at high densities (as happens shortly after an initial infection), they somehow manage to cover any new dengue virus sufficiently to disable it, enabling macrophages to kill it. If, however, the antibody concentrations are in what Harris describes as a “danger zone”—not low and not high—they may facilitate the virus’s entry into the macrophages without disarming it, thereby accelerating virus production.
Harris’s Science paper describing these results was, in the words of Jean Lim, a virologist at the Icahn School of Medicine at Mount Sinai, a “rock star study” that swayed some of the staunchest naysayers of ADE. Her unexpected findings may also have hit on the solution to the dengue vaccine mystery.
A red flag
Coincidentally, days after Harris’s paper was published in November 2017, Sanofi Pasteur made the announcement that enraged Filipino parents: do not get Dengvaxia if you have not had dengue. A month later the WHO followed suit, stating that only individuals who were proved to already have had dengue should be given the vaccine.
That was exactly what Halstead had been saying since March 2016, when he published an analysis in Vaccine arguing that Dengvaxia might cause harm. Perhaps in people who had never had dengue, the vaccine was acting like a first dengue infection, priming the body with just the right quantity of Trojan-horse antibodies to help a real infection turn severe. Young children were less likely to have already encountered dengue, and for them, the vaccine was more likely to act as a first infection. They were also more likely than adults to develop severe dengue after a second infection (as Halstead and others observed when a second dengue virus invaded Cuba in 1981). The problem was, there was no simple way to tell which children were dengue-negative before they received Dengvaxia—because Sanofi Pasteur had not collected those data for all of them before vaccinating them.
“I hate to say I told you so,” Harris says. “But we saw this coming.” At meetings and over long conference calls, she had informed Sanofi Pasteur researchers that they were not collecting the kind of data that could gauge the vaccine’s potential to put lives at risk. Instead of testing all children for prior dengue infection before they received Dengvaxia, Sanofi Pasteur tested only 10 to 20 percent of them. The company argues that it was forging through unchartered territory using the best protocols known to vaccine science. “It’s routine in many vaccine trials to bleed only 10 to 20 percent of participants,” says Su-Peing Ng, global medical head at Sanofi Pasteur.
After the disturbing hospitalization rate came to light, the researchers could not go back and bleed the thousands of children in the clinical trials to check their dengue status prior to vaccination. It was too late—they had already been vaccinated. Sanofi Pasteur worked with scientists at the University of Pittsburgh to develop a novel assay that could test the vaccinated children for evidence of prior dengue infection. That reassessment was the basis for the company’s November 2017 warning that only those who had had dengue before should receive Dengvaxia.
The earlier recommendations had been based on the preliminary findings from the clinical trials, which showed that Dengvaxia was safer for older children. As the new tests revealed, however, age served in part as a proxy for prior dengue infection. Nine-year-olds are more likely than toddlers to have already had a dengue infection, especially in places where dengue is endemic, so giving the vaccine to them should be, on average, safe. But neither age nor endemicity is a surefire way of knowing whether a child has had dengue: the only way to know for certain is through a blood test. “Mixed in with a group of nine-year-olds will always be some kids who have never had dengue,” Halstead says.
Halstead had very publicly let the WHO know about his concerns. In a December 2016 paper in the Journal of Infectious Diseases, he stated that a claim made by the WHO’s principal advisory group on vaccines was wrong. The group had said that the risk of hospitalization for kids aged two to five peaks in the third year after vaccination and then “dissipates.” Halstead argued that longer-term results from Sanofi Pasteur’s clinical trials refuted this assertion. Independently analyzing the clinical trial data, Dans, Dans and others argued in a paper in the Journal of Clinical Epidemiology that there was “no biological basis for a threshold age of 9 years” beyond which Dengvaxia could be assumed to be safe.
The WHO stands by its decision to recommend the vaccine for older children who live in countries hardest hit by dengue, however. “The review done was extremely thorough, transparent and according to our published procedures,” says Joachim Hombach, senior health adviser in the WHO’s department of immunization, vaccines and biologicals. “Different options of possible recommendations were discussed, and the one published in 2016 was the consensus position of the advisory committee.”
Ongoing controversy
In July 2018 Sanofi Pasteur published its reanalysis of clinical trial data using the Pittsburgh test in the New England Journal of Medicine. The review confirmed a higher risk of severe disease and hospitalization in “seronegative” children (those who had no evidence of prior dengue infection in their blood) who had received the vaccine, compared with those who had not. The “vaccine partially mimics primary infection and increases the risk of severe dengue during subsequent infection,” the researchers wrote. Although ADE advocates had predicted this finding, the paper said that the “immunopathogenic mechanisms underlying these findings remain unknown.”
Halstead contends that Sanofi Pasteur researchers are in “denial” about the evidence from their own trials. Ng counters that exactly how ADE boosts infection has yet to be demonstrated in humans. “ADE is more of a lab observation, an in vitro observation. We’ve not seen it clinically proven in humans,” she says. “We don’t know if the underlying mechanism is ADE or not.” The overall impact of Dengvaxia on public health remains beneficial, Ng asserts. In children who are age nine and older and who already had dengue, Dengvaxia reduces the rate of severe disease and hospitalization by around 80 percent, according to Sanofi Pasteur. (For reasons that remain unclear, two bouts of dengue appear to confer lifelong immunity to the disease. Strictly speaking, the vaccine is useful only for those who have had one bout but not two.)
Ng is not the only one who disputes that ADE is the main mechanism behind life-threatening dengue disease. Duane Gubler, founding chief of the dengue branch at the Centers for Disease Control and Prevention and an emeritus professor in the Emerging Infectious Diseases Program at Duke-NUS Medical School in Singapore, argues that DENV-2 and DENV-3 have historically been associated with outbreaks of severe disease. As such, the type of virus could be at least as important as ADE in determining the course of an infection. Alan Rothman, a professor of cell and molecular biology at the University of Rhode Island, says T cells, which recruit and activate macrophages and secrete inflammatory chemicals, are more directly involved in causing severe dengue than are antibodies. Halstead, on the other hand, regards T cells primarily as saviors. They kill dengue-infested macrophages, he says, at which time the viruses may release a protein that damages blood vessels. Doctors nonetheless can save a patient by maintaining his or her fluid levels, buying the T cells time to clean out the virus.

Vaccinated children and their parents protest the Philippines' 2016–2017 dengue immunization program. Credit: Bullit Marquez AP Photo
Toward a safer vaccine
With dengue infecting around a million people every day and popping up in places it has never been seen before, the need for a safe vaccine is becoming ever more urgent. Armed with the new information from Sanofi Pasteur, novel dengue vaccine makers are quick to say they are doing things differently. “We’ve designed our trial in such a way to ask the most important question—how does it perform in dengue naives?” says Rajeev Venkayya, president of the Global Vaccine Business Unit at Takeda Pharmaceutical Company. Takeda is currently testing its dengue vaccine in children ages four to 16 years in Latin America and Asia. “When we started this trial in 2016, we were well aware of the concern about this issue in naives,” Venkayya says. “So we made sure to have naives in our trial and collect baseline blood samples from 100 percent of participants.” In January 2019 Takeda announced preliminary results from its clinical trials: the vaccine was effective. Fully assessing safety will likely take more time, however.
At least two other dengue vaccines are being developed, one by the National Institutes of Health and one by GlaxoSmithKline. They are years from being licensed—if they are found to be safe and effective. Gubler says that any vaccine will likely protect well against a couple of dengue viruses but not so well against the others. “And that being the case, there’s always a risk of ADE,” he continues. “So do we use those vaccines, or do we shelve them and wait another 50 years for a perfect vaccine?” Halstead is far more optimistic. “There’s a really good vaccine out there,” he says—the NIH vaccine, which, he wrote in a paper, “has met virtually all of the goals needed to demonstrate preclinical efficacy and safety for humans,” even if it has yet to undergo extensive clinical trials.
The FDA’s October 2018 announcement that it would expedite review of Dengvaxia has added fresh urgency to this debate. The burden of dengue disease in the U.S. is in territories such as Guam, the U.S. Virgin Islands, Samoa and Puerto Rico, where Gubler was based as chief of the CDC’s dengue branch. He supports the use of the vaccine in places such as Puerto Rico, where, he says, the dengue surveillance system is far more robust than in the Philippines. That is, medical practitioners there should be able to keep tabs on vaccinees and ensure prompt hospitalization if they develop signs of serious disease. “I’m in favor of using it in highly endemic areas without pretesting because I think with good disease surveillance and case management, the risk of ADE is minimal,” Gubler says.
Halstead disagrees: “This is a harmful product unless administered only to proven seropositive individuals.” But proving previous dengue infection requires lab testing, which is not always available in many parts of the world with dengue epidemics. Controversially, the WHO advised in September 2018 that although prior screening for dengue infection was preferable, when such testing was not feasible, countries could nonetheless consider administering Dengvaxia in populations with 80 percent or higher dengue endemicity for those age nine and older. Asked to explain the ethical rationale for this recommendation, Hombach stated that the WHO had carefully weighed the pros and cons; it had also noted that such a campaign should be accompanied by “full disclosure of the risks of vaccination of persons with unknown serostatus.” Effectively explaining such complex issues in ethnically diverse countries, where many people may not comprehend the languages that health officials speak or be able to read information sheets could, however, be a challenge. Sanofi Pasteur takes a more cautious view. Spokesperson Karen Batoosingh says that “the vaccine should be available for people with a prior infection to prevent against subsequent infections” and that the company is striving to develop “a new rapid dengue test to ensure broader access to the vaccine for all those who could benefit from its protective value.”
Loss of trust
The repercussions from the vaccination program are still reverberating across the Philippines. Speaking before a senate inquiry panel, Aquino explained that dengue incidence in the country had been increasing at an alarming rate, and he had hoped that Dengvaxia could prevent the virus from invading densely populated urban areas. By this past February, however, both the senate and the house of representatives had recommended that Aquino, Garin and other senior officials be charged under an antigraft law for irregularities in the procurement and administration of the vaccine. The families of nearly three dozen dead children have brought criminal cases against Garin and other Filipino officials, accusing them of reckless imprudence amounting to homicide and torture. (Asked to comment on the circumstances in which the vaccination campaign was rolled out, Undersecretary of Health Enrique Domingo stated that he had stepped into the position in December 2017, after the uproar began, and had no personal knowledge of what had taken place.)
Amid the fear and suspicion, several outbreaks of measles have crept across the Philippines. In February, the country reported that more than 8,400 have become sick and more than 130 have died. Parents were too frightened to vaccinate their kids. According to a study by the London-based Vaccine Confidence Project, in 2018 fewer than a third of Filipinos strongly agreed that vaccines are important, down from 93 percent in 2015. In that study, published in Human Vaccines & Immunotherapeutics, Heidi Larson, the project’s director, and her co-authors argued that “biased media hype”—in particular, “false narratives aiming to vilify authorities, scientists and regulators” and “senate and congress inquiries that resembled the inquisition”—had prompted public panic and loss of trust in vaccines. Dans, Dans, Halstead and others teamed up to respond that several factors had contributed to the decline in public confidence, not least Sanofi’s “exaggerated” claims of the safety of Dengvaxia: “The outrage was a result of the loss of trust rather than its cause.”
Asked by Scientific American if he was giving ammunition to antivaxxers, Halstead responded that he had co-founded the Children’s Vaccine Initiative in the 1990s, which later morphed into Gavi, a global public-private partnership that strives to improve vaccine access for children in poor countries. “I have very strong bona fides as a supporter of vaccines and vaccination,” he says.
Even as the scientists battle it out, the parents of the vaccinated children are suffering sleepless nights, according to Antonio Dans. “The mothers are really distressed about, Was my child seronegative when he was vaccinated? Why weren’t we told it could be harmful? They call us and say, My child has a cough, should we rush him to the hospital? He seems to me slightly febrile, should he go to school?” he relates. “And how do you monitor a cold and a fever in [roughly] a million kids and find out if it’s dengue or not? That’s a logistical nightmare, and that’s what we were warning DOH about.” Virtually every death in the vaccinated group was being blamed on Dengvaxia, even if it was clearly unrelated, he adds—and much of this rage and turmoil could have been avoided by accurate and timely scientific advice from trusted authorities. “So that’s the sad thing here—that the WHO added to the confusion,” Dans concludes.
Halstead worries that as antibody levels in the vaccinated seronegative wane with time, to an intermediate level where ADE becomes more likely, they will become increasingly predisposed to developing severe dengue when they do experience an actual infection. Using Sanofi Pasteur’s figures from the clinical trials—that five out of every 1,000 seronegative vaccinated children were hospitalized for dengue, of whom two had severe dengue—he calculated that more than 4,000 children could be hospitalized for vaccine-enhanced dengue disease in the Philippines. “I rub my eyes at what’s happening,” he says. “Why isn’t Sanofi spending a lot of time thinking, ’Okay, now that we’ve sensitized so many people [to ADE], how are we going to protect them?’” Asked this question, Ng responded that it was unclear whether the cases of severe dengue in the vaccinated group arose from vaccine failure or ADE. All patients, regardless of whether they had dengue before or had been vaccinated or not, should guard against mosquito bites, be monitored for early signs of dengue disease, and seek prompt treatment on indications of more severe disease. Asked when the final report from the clinical trials would be published, Sanofi Pasteur responded that the outcomes had been displayed on a poster at a meeting of the American Society of Tropical Medicine and Hygiene in late 2018.
Vaccines have saved uncountable lives. Naturally occurring smallpox has been wiped off the face of the planet, and polio has almost been vanquished; tetanus and rabies no longer inspire terror. Despite these achievements, public fear of vaccines has been growing, placing millions of children at risk of avoidable disease. The increasing skepticism about vaccines is almost entirely the result of misinformation. Even so, the twists and turns of the Dengvaxia story complicate the usual narrative of valiant scientists battling public ignorance and prejudice in the quest to keep everyone safe.
The dengue saga also raises difficult questions about how pharmaceutical companies and regulators should proceed in the context of evolving scientific knowledge and imperfect vaccines. Is it ethical to endanger a minority in the interest of protecting a majority, as the WHO’s September 2018 advisory on Dengvaxia implies? Who should be making these difficult decisions: global bodies of experts, national health authorities, fully informed parents and doctors, or some combination of these? And who should be held accountable when things go wrong?
Editors' Note: Since this article was published in April, there have been additional deaths from measles—724 total, from all of 2018 until June 29, 2019, according to the Red Cross. In May of this year, the FDA approved Dengvaxia for use in individuals ages nine to 16. Sanofi Pasteur has still not released results from its clinical trials.

