14/05/19
Eliminating malaria demands quality drugs

Send to a friend
The details you provide on this page will not be used to send unsolicited email, and will not be sold to a 3rd party. See privacy policy.
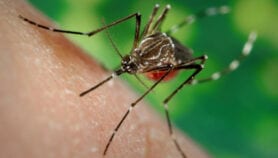
Fighting malaria calls for strict quality control over drugs used against the parasite to prevent resistance.
From the cobblestone streets of Paris, France to the bustling streets of Abuja, Nigeria, health experts around the world gathered on World Malaria Day (25 April) and issued a collective cry for more action to eliminate malaria.
This year, I joined leaders from across the Greater Mekong Sub-region (GMS) in Bangkok to take stock of progress against our joint commitment to eliminate malaria in the Asia Pacific by 2030. Through better cross-border collaboration and improvements in data sharing, we have witnessed remarkable progress; in the GMS alone, cases are down by more than 75 per cent since 2012.
While this success is worthy of celebration, the region is also coming to grips with a growing challenge to this progress: drug-resistant malaria.
“Timely data sharing will allow us to catch spurious antimalarials before they reach our hospitals, health centres and market stalls”
Sivong Sengaloundeth, former deputy director of the Food and Drug Department in Lao PDR
Historically, the GMS has been a hotbed for resistance. In the 1960s and 1970s, the malaria parasite developed immunity to widely-used medicines like chloroquine before spreading to other regions of the world. Now history may be repeating itself as the parasite develops resistance to one of today’s most effective antimalarials — artemisinin.
So, how is it that the parasite seems to be outsmarting our drugs? One answer may be hiding in our antimalarials themselves.
Resistance to artemisinin was detected in the GMS around the same time researchers discovered that between 38 and 90 per cent of sampled artemisinin medicines were substandard or spurious. The medicines did not contain enough active ingredients to effectively kill the malaria parasite, which allowed it to develop immunity to the medicine.
Consider a woman who develops symptoms of malaria and is given artemisinin. After a few days of treatment, she feels no better. Although she has a job, home responsibilities and little time to spare, she has no choice but to return to the clinic where she receives a second-line drug. She has now paid for medicine twice out of her own pocket. The alternative treatment brought improvement, but the cost has been high — in both time and money.
Sadly, this woman’s experience has become all too common, and the widespread use of substandard medicines is one of the reasons for this growing problem. In South-East Asia alone, it was estimated that up to 35 per cent of antimalarial drugs in circulation were of poor quality. The WHO estimates that across low- and middle-income countries, nearly one in five antimalarials are poor quality and some 200,000 people die every year due to these drugs.
During my tenure at the Food and Drug Department (FDD) in the Lao People’s Democratic Republic (PDR), I witnessed medicine quality issues at all levels of the health system — from manufacturing to delivery. The FDD addressed this by conducting special trainings for pharmacists to raise awareness about the problem of poor-quality medicines. We also equipped pharmacists with MinilabsTM — self-contained kits for on-the-spot quality testing — that enabled them to test medicines in their own clinics or pharmacies. In less than a decade, we reduced the proportion of poor-quality antimalarials in circulation from 84 per cent to 25 per cent.
Across the region, I have also been encouraged to seek political support to tackle this problem. Last year, Cambodian Prime Minister Hun Sen and other leaders from across the GMS pledged commitment to improve access to quality medicines. It’s essential that such commitments are coupled with sustained actions to ensure that every malaria patient receives medicines that work.For one, countries should ensure that the medicines imported or purchased for their health systems are quality-assured.
Government agencies — including health, customs and justice departments — must work across national boundaries to share issues in real time. Timely data sharing will allow us to catch spurious antimalarials before they reach our hospitals, health centres and market stalls. Consistent data collection will help inform solutions and policy decisions. But these efforts will require more sustained funding from donors and countries alike.
Finally, physicians, pharmacists and health workers who are on the frontlines of malaria response must be equipped with the training and tools they need to detect fake medicines before they ever reach our patients’ hands — just as we did in Lao PDR.
The threat of resistance looms large, and the GMS has served as the harbinger of what is to come. By safeguarding our region against poor-quality antimalarials, we can carry forward the promise of malaria elimination not just at home but around the world.
Sivong Sengaloundeth, a former deputy director of the Food and Drug Department in Lao PDR is a champion of the Meds We Can Trust Campaign, a global movement to raise awareness about the importance of quality medicines and inspire collective action.
This piece was produced by SciDev.Net’s Asia & Pacific desk.